
At Prix Pharmaceutica, we manufacture high-quality, optimized veterinary medicines. Our GMP-compliant production facility is located in Pharmacity, Lahore, Pakistan. The facility spans 90,000 sq. ft., with 25,000 sq. ft. of covered space.
Manufacturing
We strive to manufacture high-quality, optimized medicines, metting global, scientific, and best practice standards. Endeavouring to stay at the cutting edge of global technological development, we strive to provide our customers with the best, innovative, and modern therapies available.
Our cGMP- and ISO-compliant production facility is located in Pharmacity, a pharmaceutical industrial zone located in Lahore, Pakistan. The facility spans 90,000 sq. ft., with 25,000 sq. ft. of covered space, and is equipped to manufacture both sterile and non-sterile dosage forms.
Manufacturing


Our Philosophy
At Prix, we appreciate the fact that highly-specialized manufacturing and production can only be possible through knowledgeable, well-trained, and skilled professionals. Therefore, our topmost priority is to have the best, most capable professionals and workers in the industry to boost and manage our pharmaceutical production.
Manufacturing Sectors
Oral Liquids
Bolus and Granulation
Water Soluble Powders (WSP)
Antibiotic WSP
General Antibiotic Powders
Penicillin Antibiotic Powders
Penicillin Injections
Injectable Liquids
Solutions and suspensions manufactured under sterile, GMP-compliant conditions.
Therapeutic blends for bacterial infections in various species.
Compressed formulations for large animals with consistent release properties.
Non-penicillin powders for broad-spectrum antibacterial coverage.
Dedicated lines for β-lactam antibiotics ensuring cross-contamination control.
Easily mixable powders for poultry and livestock administration.
Sterile injectable formulations in glass vials for quick action.
Powder and liquid injectables made under strict environmental controls.

Research and Development


Our team is on the continuous hunt for the best sources of inputs, better and improved formulations, and new and improved process, all of which are instrumental in pushing us towards innovation and improvement.
Quality Control & Quality Assurance
At Prix, we take full responsibility for our products and services. We do not stop at just manufacturing the best — we maintain strict monitoring of every process and product across its entire lifespan. Together, this ensures we consistently deliver on our ambitious goals and product claims.
Quality Control (QC)
Our in-house QC department is equipped with advanced analytical technologies and operates under strict protocols to ensure the quality, safety, and efficacy of all products.
Raw material, in-process, and finished product testing
Use of HPLC, UV spectrophotometers, and microbiological testing tools
Environmental and stability monitoring
Dedicated protocols for antibiotic and penicillin product lines
Full traceability and batch-level validation
Quality Assurance (QA)
QA at Prix goes beyond paperwork. Our team enforces extensive and robust process controls, regulatory alignment, and continuous improvement through the following:
Implementation of cGMP-based standard operating procedures (SOPs)
Document control and batch record verification
Deviation tracking and CAPA systems
Internal inspections and compliance audits
Staff training and performance tracking
Our in-house QC department is equipped with advanced analytical technologies and operates under strict protocols to ensure the quality, safety, and efficacy of all products.
QA at Prix goes beyond paperwork. Our team enforces extensive and robust process controls, regulatory alignment, and continuous improvement.
Certifications






ACS Registrars Pakistan
(ISO Certificate)
Government of Pakistan
(License to Manufacture)
Drug Regulatory Authority
(cGMP Certificate)
Prix has developed and implemented a quality-focused management system that aligns with global standards and regulatory expectations. Our commitment to quality is reflected in our certifications.
Self Inspection & Auditing
Proactive internal inspections help ensure that quality is embedded throughout the organization, fostering a culture of continuous improvement.
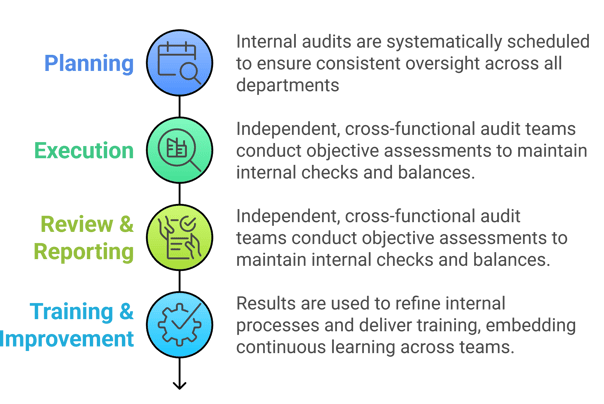
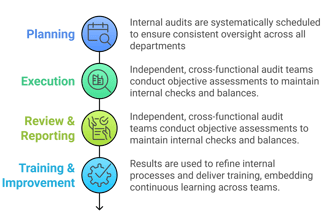
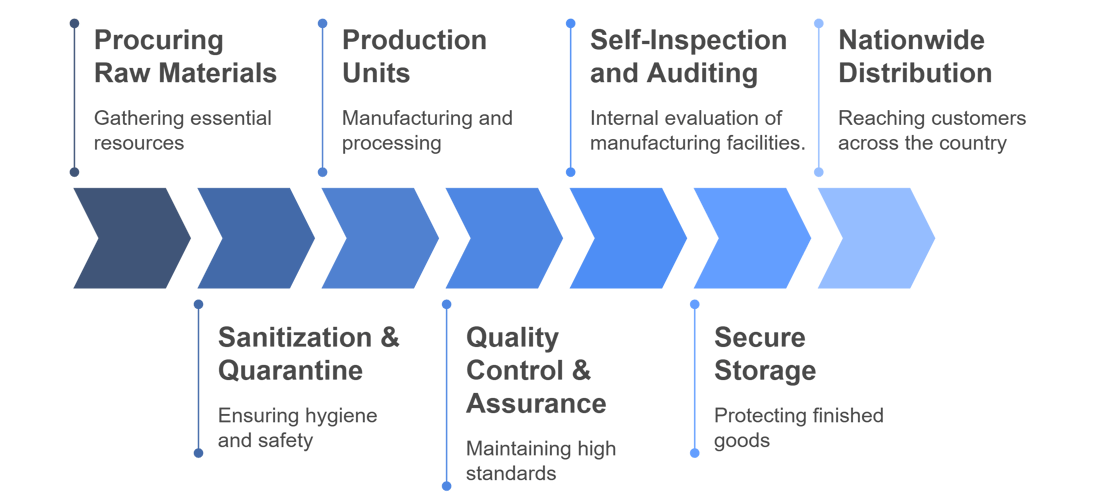
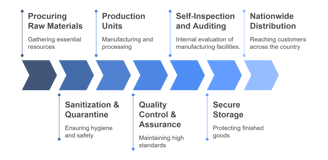
Our Structured Approach to Manufacturing
Featured Products
Manufactured Right Here at Prix Pharmaceutica
Contact
Manufacturing Plant:
+92 42 35975322
© 1991. All rights reserved.


Head Office:
+92 42 36283464
+92 42 36363055
